ABOUT THE GLUMETZA LAWSUIT
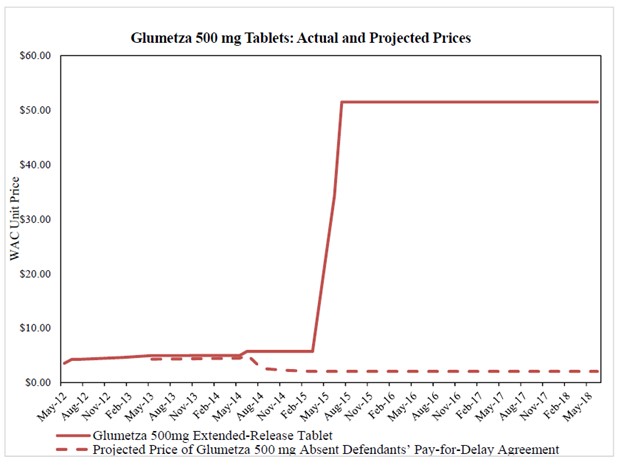
The suit alleged Assertio and Bausch paid Lupin to delay entry and promised in return not to compete with an authorized generic for the first twelve months that Lupin’s generic was on the market. As a result, Glumetza purchasers – pharmaceutical wholesalers, pharmacies, insurance companies, and consumers – paid far more for brand Glumetza and, once it finally launched, generic Glumetza. By the end of July 2015, before Lupin launched its generic, Bausch had spiked the price of Glumetza by more than 800 percent.
Glumetza’s developer, Assertio, obtained several patents that it claimed cover Glumetza. In 2009, Lupin, the first company to file an Abbreviated New Drug Application (ANDA), sought FDA approval to market its generic version of Glumetza.
As the first filer, Lupin would have been statutorily entitled to 180 days as the only ANDA generic on the market upon FDA approval, though Assertio could launch an “authorized generic” – its brand drug under a generic label – and compete during those 180 days, resulting in lower prices for purchasers. Assertio sued Lupin for patent infringement, alleging Lupin would infringe its Glumetza patents. On Feb. 22, 2012, Lupin and Assertio (along with Santarus who had assumed responsibility for the commercialization of Glumetza) settled, with Assertio/Santarus paying Lupin to delay entry until February 2016 and Assertio/Santarus agreeing not to market an authorized generic for a year after Lupin launched. Lupin could charge higher prices without generic competition, at the expense of purchasers.
In mid-2015, before generic competition began, Bausch (having acquired Santarus) initiated price increases totaling approximately 800 percent. What had been a $6 tablet suddenly exceeded $50. And when Lupin entered, it took advantage of Glumetza’s high price, pricing just below Bausch until later generics entered and brought the price down.
In September 2021, Hagens Berman secured a settlement with Bausch Health Companies Inc., Salix Pharmaceuticals, Ltd., Salix Pharmaceuticals, Inc., Santarus, Inc., Assertio Therapeutics, Inc., Lupin Pharmaceuticals Inc. and Lupin Ltd. totaling $453.85 million on behalf of direct purchasers of Glumetza, resolving these allegations against the defendants.
TOP PHARMA LAW FIRM
Hagens Berman is one of the most successful litigation law firms in the U.S. taking on pharmaceutical companies and has achieved more than $325 billion in settlements against Big Pharma largest sellers and manufacturers for antitrust schemes, pay-for-delay, IP shams and other forms of wrongdoing that drive up the costs of prescription drugs for consumers and others.
CASE TIMELINE
Hagens Berman is pleased to announce settlements with Bausch Health Companies Inc., Salix Pharmaceuticals, Ltd., Salix Pharmaceuticals, Inc., Santarus, Inc., Assertio Therapeutics, Inc., Lupin Pharmaceuticals Inc. and Lupin Ltd. totaling $453.85 million. These settlements provide significant relief to direct purchasers of Glumetza, resolving allegations against the defendants that direct purchasers overpaid for brand and generic Glumetza due to an alleged conspiracy to suppress generic competition of the diabetes drug. Hagens Berman will continue to litigate on behalf of the class to recover further compensation from remaining defendants.
Judge Alsup preliminarily approved all three settlements on September 22, 2021. The final fairness hearing will be held on January 20, 2022.
Notice of class certification was sent to members of the direct purchaser class via mail and e-mail.
The Court approved the proposed class notification plan.
The Court certified the class of direct purchasers who purchased brand and generic Glumetza during the May 6, 2012 through August 15, 2020 period and appointed Hagens Berman Co-Lead Class Counsel, with Steve Shadowen of Hilliard & Shadowen and Joseph Vanek of Sperling & Slater.
After the Court granted leave to amend, HBSS filed a first amended consolidated complaint on behalf of the proposed direct purchaser class.
The Court denied defendants’ motion to dismiss as to the direct purchasers’ claims.
The court appointed Hagens Berman Interim Co-lead Counsel.
HBSS filed a consolidated amended complaint on behalf of a proposed class of direct purchasers of brand and generic Glumetza.