Did you buy insulin after 2019 either with co-insurance or at full price? You may have rights due to an alleged overpricing scheme regarding Apidra, Basaglar, Fiasp, Lantus, Levemir, Novolog, Humalog and/or Toujeo.
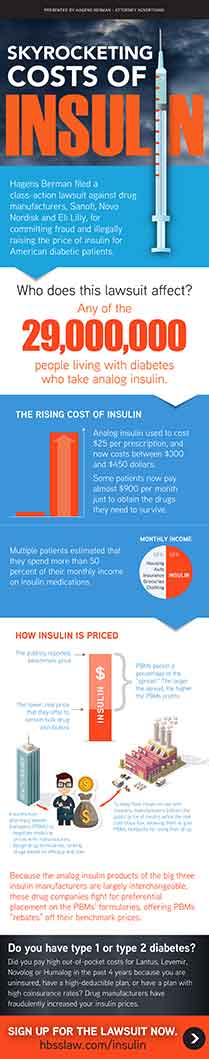
According to the lawsuit, in recent years, Big Pharma has sought to exponentially increase its profits on drugs that keep people alive, raising costs to consumers that are unrelated to any increase in production or research and development costs. Insulin has allegedly been a prime example of such conduct.
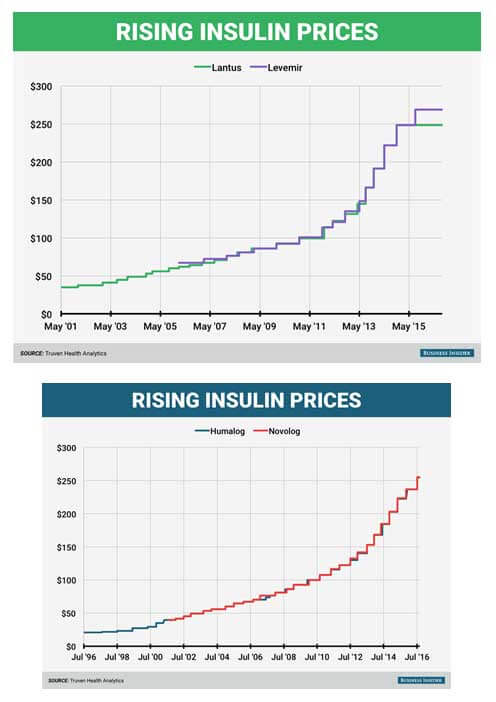
The firm’s investigation indicated that these companies allegedly increased the publicly reported list prices of Lantus, Levemir, Novolog, Apidra and Toujeo by more than 160% in the last five years, while keeping the prices they offer to pharmacy benefit managers constant or even lowering them.
DRUG-PRICING FRAUD EXPLAINED
According to the lawsuit, in the drug pricing and distribution system, drug manufacturers often offer slightly lower prices to pharmacy benefit managers. However, attorneys say that in the case of Lantus, Levemir, Novolog, Apidra, Basaglar, Fiasp and Toujeo, the difference between the drugs’ list prices—the price on which consumer payments are based—and the prices offered to pharmacy benefit managers is enormous. Indeed, according to the complaint, this “spread” between prices is one of highest in the entire pharmaceutical industry.
Hagens Berman’s complaint alleges that this difference in pricing — between the price on which consumers are forced to pay and that which industry insiders pay — is so enormous as to render the list prices on which consumer pay fraudulent. According to the lawsuit, as the list prices of Lantus, Levemir, Novolog, Apidra, Basaglar, Fiasp and Toujeo move further and further away from the real prices offered to pharmacy benefit managers, these list prices become so misrepresentative, they are fraudulent. The lawsuit seeks damages on behalf of people living with diabetes who allegedly overpaid for these life-saving insulin medications because they purchased these drugs based on the list prices the defendant drug companies set.
HAGENS BERMAN'S EFFORTS
In September 2017, Hagens Berman was appointed Interim Lead Counsel to direct this lawsuit. In 2023, the firm reached a settlement agreement with defendant Eli Lilly valued at more than $500 million.
The discovery process is ongoing with the remaining defendants. Sanofi and Novo Nordisk are in the process of producing records of their conduct to the plaintiffs and Hagens Berman is reviewing that production to build its case.
YOUR RIGHTS
The lawsuit seeks reimbursement for the allegedly fraudulent activities carried out by some of the largest makers of analog insulin drug products – Sanofi, Novo Nordisk and Eli Lilly.
Hagens Berman believes action must be taken for the millions of people living with diabetes who are allegedly forced to pay fraudulently high prices for analog insulins or else forgo purchase of these medications at the whim of Big Pharma.
CASE TIMELINE
Hagens Berman Sobol Shapiro transferred the Insulin Class Action on behalf of patients paying high out-of-pocket costs for analog insulin to the District of New Jersey from the District of Massachusetts because there was a related case ongoing in the District of New Jersey. Efficiency concerns led us to transfer the case from the District of Massachusetts to that of New Jersey. Absent such a move it is likely the case would have been subject to an MDL petition, which would have slowed the case’s progress.
James Cecchi, of Carella, Byrne, Cecchi, Olstein, Brody & Agnello, P.C., filed a related suit on behalf of an investor class against Novo Nordisk in New Jersey. James Cecchi remains as one of the lawyers in that suit. There will be a lead counsel selection process pursuant to the federal law governing securities cases in that lawsuit. Hagens Berman Sobol and Shapiro is not involved in that investor class lawsuit.
Hagens Berman Sobol Shapiro is proposed lead counsel for the patient-plaintiff class. We will continue to represent only this patient class, and advocate in the best interests of this class only. As of right now, James Cecchi is local counsel for the patient-plaintiff class. However, because Hagens Berman Sobol Shapiro is lead counsel, our firm will be directing the course of this litigation. We will do so in the best interests of the patient-plaintiff class, and that class only.
The United States Senate Finance Committee release a report substantiating and agreeing with our complaint. Indeed, this Senate Finance Committee investigation tracked that initiate by Hagens Berman.